Role of Adenosine in PD
There is more to Parkinson’s disease (PD) than just dopamine1
Both dopamine and adenosine regulate movement in PD1
Stimulation of dopamine receptors is like pressing the gas pedal of a car, which initiates movement, while
stimulation of adenosine A2A receptors is like applying the brake, which suppresses movement.2-4
In PD, motor dysfunction occurs when there is a deficiency of dopamine and an overactivation of adenosine A2A receptors.
Levodopa/carbidopa acts on the gas but not the brake.2-4
Understanding the go (direct) and no go (indirect) pathways in PD2
Imbalance among these pathways contributes to motor dysfunction5
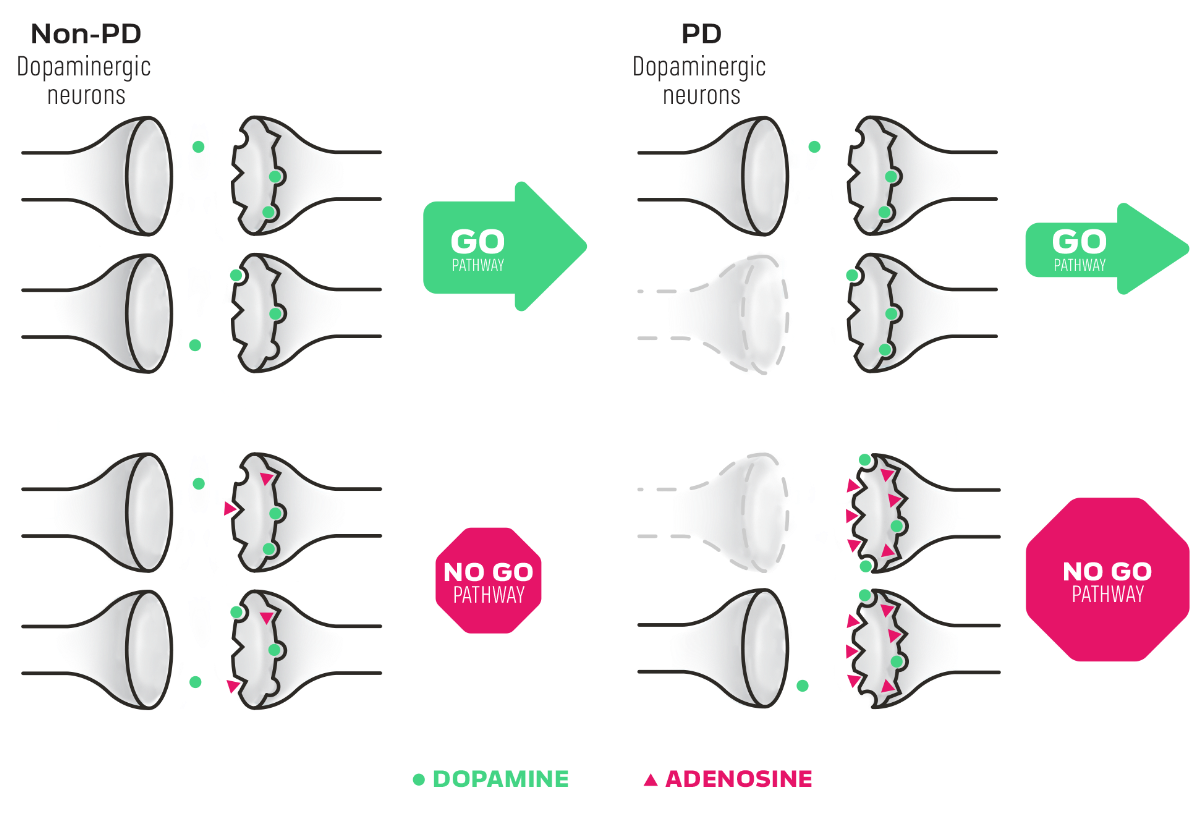
- Dopamine works in the go and no go pathways, while adenosine works in the no go pathway only2,5
- In normal movement, balance between the pathways facilitates movement signals from the basal ganglia, enabling motor activity2,4
- In patients with PD, dopamine levels are decreased, reducing the activity of the go pathway and increasing the activity of the no go pathway2,4
- As PD progresses, adenosine A2A receptor concentration increases and the effect of adenosine on the no go pathway becomes more prominent6
Human brain PET image by A2A receptor tracer7
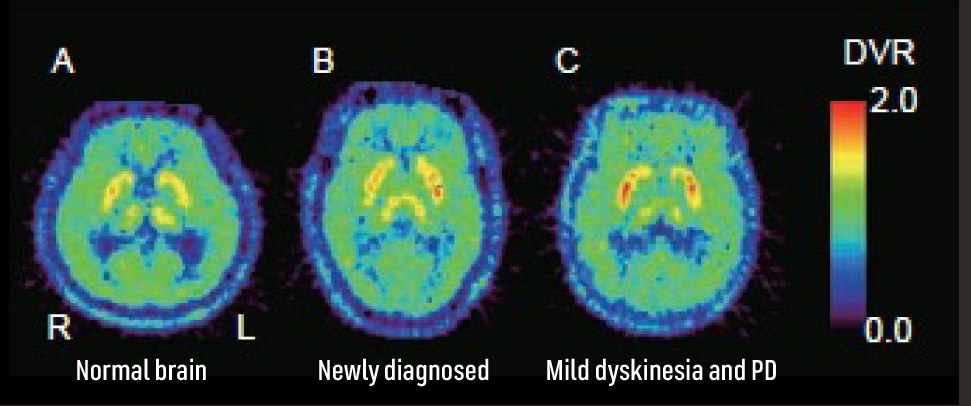
Adapted from Mishina M, Ishiwata K. Adenosine receptor PET imaging in human brain. Int Rev Neurobiol. 2014;119:51-69.
The density of adenosine A2A receptors is identified by 11C-TMSX PET imaging.7
A: The normal subject is a healthy, 56-year-old man.
B: The newly diagnosed patient with Parkinson’s disease is a 56-year-old man with left-dominant parkinsonism. The distribution volume ratio (DVR) of 11C-TMSX was smaller in the right putamen than in the left.
C: The patient with mild dyskinesia and Parkinson’s disease is a 66-year-old woman with left-dominant parkinsonism. Compared with the normal subject and newly diagnosed patient, the DVR of 11C-TMSX in the striata was increased in the patient with dyskinesia.
Around 1 million people in the United States are living with Parkinson’s8
Within 5 years of initiating levodopa/carbidopa, 50% of patients with PD experience “off” time, including motor complications.9

Patients taking levodopa/carbidopa to control symptoms of PD may still experience “off” time.10

Actor Portrayals.
Actor Portrayals.
Indication
NOURIANZ® (istradefylline) is an adenosine receptor antagonist indicated as adjunctive treatment to levodopa/carbidopa in adult patients with Parkinson’s disease (PD) experiencing “off” episodes.
Important Safety Information
Warnings and Precautions
Dyskinesia: NOURIANZ in combination with levodopa may cause dyskinesia or exacerbate pre-existing dyskinesia. In clinical trials, 1% of patients treated with either NOURIANZ 20 mg or 40 mg discontinued treatment because of dyskinesia, compared to 0% for placebo.
Hallucinations / Psychotic Behavior: Because of the potential risk of exacerbating psychosis, patients with a major psychotic disorder should not be treated with NOURIANZ. Consider dosage reduction or discontinuation if a patient develops hallucinations or psychotic behaviors while taking NOURIANZ.
Impulse Control / Compulsive Behaviors: Patients treated with NOURIANZ and one or more medication(s) for the treatment of Parkinson’s disease (including levodopa) may experience intense urges to gamble, increased sexual urges, intense urges to spend money, binge or compulsive eating, and/or other intense urges, and the inability to control these urges. In clinical trials, 1 patient treated with NOURIANZ 40 mg was reported to have impulse control disorder, compared to no patient on NOURIANZ 20 mg or placebo.
Drug Interactions
The maximum recommended dosage in patients taking strong CYP3A4 inhibitors is 20 mg once daily. Avoid use of NOURIANZ with strong CYP3A4 inducers.
Specific Populations
Pregnancy: Based on animal data, may cause fetal harm.
Hepatic impairment: The maximum recommended dosage of NOURIANZ in patients with moderate hepatic impairment is 20 mg once daily. Avoid use in patients with severe hepatic impairment.
Adverse Reactions
The most common adverse reactions with an incidence ≥5% and occurring more frequently than with placebo were dyskinesia (15%, 17%, and 8%), dizziness (3%, 6%, and 4%), constipation (5%, 6%, and 3%), nausea (4%, 6%, and 5%), hallucination (2%, 6%, and 3%), and insomnia (1%, 6%, and 4%) for NOURIANZ 20 mg, 40 mg, and placebo, respectively.
You are encouraged to report suspected adverse reactions to Kyowa Kirin, Inc. at 1-844-768-3544 or FDA at
1-800-FDA-1088 or www.fda.gov/medwatch.
Please see full Prescribing Information for NOURIANZ.
References: 1. NOURIANZ. Prescribing Information. Kyowa Kirin, Inc; 2023. Accessed November 1, 2023. https://www.nourianzhcp.com/assets/pdf/nourianz-full-prescribing-information.pdf. 2. Kalia LV, Brotchie JM, Fox SH. Novel nondopaminergic targets for motor features of Parkinson’s disease: review of recent trials. Mov Disord. 2013;28(2):131-144.
References: 1. Kalia LV, Brotchie JM, Fox SH. Novel nondopaminergic targets for motor features of Parkinson's disease: review of recent trials. Mov Disord. 2013;28(2):131-144. 2. Mori A. Mode of action of adenosine A2A receptor antagonists as symptomatic treatment for Parkinson’s disease. Int Rev Neurobiol. 2014;119:87-116. 3. Varani K, Vincenzi F, Tosi A, et al. A2A adenosine receptor overexpression and functionality, as well as TNF-α levels, correlate with motor symptoms in Parkinson’s disease. FASEB J. 2010;24(2):587-598. doi:10.1096/fj.09-141044. 4. Fuxe K, Marcellino D, Genedani S, Agnati L. Adenosine A2A receptors, dopamine D2 receptors and their interactions in Parkinson's disease. Mov Disord. 2007;22(14):1990-2017. doi: 10.1002/mds.21440. 5. Morelli M, Di Paolo T, Wardas J, Calon F, Xiao D, Schwarzschild MA. Role of adenosine A2A receptors in parkinsonian motor impairment and L-DOPA-induced motor complications. Prog Neurobiol. 2007;83(5):293-309. 6. Morelli M, Blandini F, Simola N, Hauser RA. A2A receptor antagonism and dyskinesia in Parkinson's disease. Parkinsons Dis. 2012;2012:489853. doi: 10.1155/2012/489853. 7. Mishina M, Ishiwata K. Adenosine receptor PET imaging in human brain. Int Rev Neurobiol. 2014;119:51-69. doi:10.1016/B978-0-12-801022-8.00002-7. 8. The voice of the patient: Parkinson’s disease. Silver Spring, MD: US Food and Drug Administration; April 2016. https://www.fda.gov/media/124392/download. Accessed June 11, 2019. 9. Hickey P, Stacy M. Available and emerging treatments for Parkinson’s disease: a review. Drug Des Devel Ther. 2011;5:241-254. 10. Stocchi F, Antonini A, Barone P, et al. Early DEtection of wEaring off in Parkinson disease: the DEEP study. Parkinsonism Relat Disord. 2014;20(2):204-211.
References: 1. NOURIANZ. Prescribing Information. Kyowa Kirin, Inc; 2020. Accessed April 1, 2021. https://www.nourianzhcp.com/assets/pdf/nourianz-full-prescribing-information.pdf 2. Kalia LV, Brotchie JM, Fox SH. Novel nondopaminergic targets for motor features of Parkinson’s disease: review of recent trials. Mov Disord. 2013;28(2):131-144. 3. Jenner P. Istradefylline, a novel adenosine A2A receptor antagonist, for the treatment of Parkinson’s disease. Expert Opin Investig Drugs. 2005;14(6):729-738. 4. Brichta L, Greengard P, Flajolet M. Advances in the pharmacological treatment of Parkinson’s disease: targeting neurotransmitter systems. Trends Neurosci. 2013;36(9):543-554. 5. Kaakkola S, Wurtman RJ. Effects of COMT inhibitors on striatal dopamine metabolism: a microdialysis study. Brain Res. 1992;587(2):241-249. 6. Kong P, Zhang B, Lei P, et al. Neuroprotection of MAO-B inhibitor and dopamine agonist in Parkinson disease. Int J Clin Exp Med. 2015;8(1):431-439. 7. Ossola B, Schendzielorz N, Chen SH, et al. Amantadine protects dopamine neurons by a dual action: reducing activation of microglia and inducing expression of GDNF in astroglia. Neuropharmacology. 2011;61(4):574-582. 8. Rubí B, Maechler P. Minireview: new roles for peripheral dopamine on metabolic control and tumor growth: let’s seek the balance. Endocrinology. 2010;151(12):5570-5581. doi:10.1210/en.2010-0745. 9. Gerlach M, Double K, Arzberger T, Leblhuber F, Tatschner T, Riederer P. Dopamine receptor agonists in current clinical use: comparative dopamine receptor binding profiles defined in the human striatum. J Neural Transm (Vienna). 2003;110(10):1119-1127. 10. Ishibashi K, Miura Y, Wagatsuma K, Toyohara J, Ishiwata K, Ishii K. Adenosine A2A receptor occupancy by long-term istradefylline administration in Parkinson’s disease. Mov Disord. 2021;36(1):268-269. doi:10.1002/mds.28378.
References: 1. NOURIANZ. Prescribing Information. Kyowa Kirin, Inc; 2020. Accessed April 1, 2021. https://www.nourianzhcp.com/assets/pdf/nourianz-full-prescribing-information.pdf 2. Kalia LV, Brotchie JM, Fox SH. Novel nondopaminergic targets for motor features of Parkinson’s disease: review of recent trials. Mov Disord. 2013;28(2):131-144. 3. Data on file. Kyowa Kirin Pharmaceutical Development, Inc., Princeton, NJ.
References: 1. NOURIANZ. Prescribing Information. Kyowa Kirin, Inc; 2020. Accessed April 1, 2021. https://www.nourianzhcp.com/assets/pdf/nourianz-full-prescribing-information.pdf 2. Data on file. Kyowa Kirin Pharmaceutical Development, Inc., Princeton, NJ.
Reference: 1. NOURIANZ. Prescribing Information. Kyowa Kirin, Inc; 2020. Accessed April 1, 2021. https://www.nourianzhcp.com/assets/pdf/nourianz-full-prescribing-information.pdf
Reference: 1. NOURIANZ. Prescribing Information. Kyowa Kirin, Inc; 2020. Accessed April 1, 2021. https://www.nourianzhcp.com/assets/pdf/nourianz-full-prescribing-information.pdf